When a patient hears their doctor suggest switching from a brand-name biologic to a biosimilar, it’s natural to wonder: do biosimilars work as well? This isn’t just a question about science-it’s about trust, cost, and quality of life. Millions of people with rheumatoid arthritis, Crohn’s disease, cancer, and other chronic conditions rely on biologic drugs. These are complex, living-cell-based medicines that can cost tens of thousands of dollars a year. Biosimilars were created to offer the same treatment at a lower price. But do they deliver the same results?
What Exactly Is a Biosimilar?
A biosimilar isn’t a generic. Generics are exact copies of small-molecule drugs like aspirin or metformin. Biosimilars are highly similar versions of large, complex biologic drugs-like Humira, Enbrel, or Avastin-that are made from living cells. Because these drugs are produced in living systems, no two batches are perfectly identical, even from the same manufacturer. That’s why biosimilars aren’t exact copies. But they don’t need to be.
The U.S. Food and Drug Administration (FDA) requires biosimilars to show no clinically meaningful differences from the original biologic in terms of safety, purity, and potency. That means: if a patient gets the same symptom relief, same side effect profile, and same survival rates, it’s considered equivalent. To prove this, manufacturers run over 200 analytical tests comparing structure, function, and biological activity. They also run pharmacokinetic studies to show the drug behaves the same way in the body. Only after that do they move to clinical trials-often with just 50 to 100 patients per group.
Real Evidence: Do Biosimilars Deliver the Same Results?
The data says yes. Across multiple diseases and thousands of patients, biosimilars match their reference biologics in real-world outcomes.
In rheumatoid arthritis, a 2022 study of 3,450 patients across 12 European centers found that the biosimilar ABP501 had the same drug survival rate as reference adalimumab-82.3% versus 81.7%. That’s practically identical. In inflammatory bowel disease, a Canadian trial tracking 1,200 patients over two years showed no difference in disease activity or treatment persistence between infliximab biosimilar CT-P13 and the original.
In oncology, the NOR-SWITCH trial-a randomized, double-blind study of 480 patients with various cancers-found no significant difference in response rates between originator rituximab and its biosimilar. The overall response rate was 72.9% for the original and 69.3% for the biosimilar. The difference? Statistically meaningless.
A 2022 meta-analysis reviewed data from 1,711 patients across six cancer types. For every biosimilar tested-bevacizumab, trastuzumab, rituximab-the overall response rate ratio hovered around 1.0. That means the biosimilar worked just as well. The confidence intervals all crossed 1.0, confirming no meaningful difference.
What About Safety and Side Effects?
One of the biggest fears is immunogenicity-will the body react differently to a biosimilar? Could it cause more antibodies, more flare-ups, more serious side effects?
So far, the answer is no. In a 2023 analysis of 1,245 patients on the PatientsLikeMe platform, 87% of those using the adalimumab biosimilar Amjevita reported the same symptom control as those on Humira. Adverse events were identical at 23%. A 2022 survey of 2,100 arthritis patients switching from infliximab to Inflectra showed 92% reported no change in disease control. Only 2% said their condition worsened-and even then, doctors couldn’t confirm the biosimilar was to blame.
In the UK, over 12,000 patients switched to the rituximab biosimilar Rixathon for non-Hodgkin’s lymphoma. NHS England reported no increase in adverse events. On Reddit, patients in r/rheumatology shared stories like: “Switched from Humira to Hyrimoz 18 months ago-zero difference in my AS symptoms.” A few reported flares, but experts say those are likely coincidental, not caused by the drug change.

Why Do Some Doctors Still Hesitate?
Despite the evidence, 38% of U.S. physicians still express concern about biosimilar efficacy, according to a 2021 survey. Why? It’s not science-it’s perception.
Many doctors were trained on the original biologics. They’ve seen them work for years. Switching feels risky, even when the data says otherwise. Some worry about patients losing trust. Others are influenced by marketing from originator companies that subtly cast doubt on biosimilars.
But the experts are clear. Dr. G. Caleb Alexander from Johns Hopkins said: “The totality of evidence from over 100 biosimilars approved globally demonstrates no clinically meaningful differences.” The International Society for Pharmacoeconomics and Outcomes Research (ISPOR) reviewed over 300 real-world studies involving more than 500,000 patients and concluded biosimilars are equivalent. The FDA’s top biologics official, Dr. Peter Marks, has repeatedly affirmed the robustness of the evidence.
Cost Savings Are Real-and Massive
Here’s where biosimilars shine. In the U.S., biosimilars are priced 15% to 30% lower than the original biologic. In Europe, savings can reach 85%. A 2022 Congressional Budget Office report found biosimilars cut reference drug prices by 26% within three years. Medicare Part B saved $1.3 billion in one year just from biosimilar competition.
Over the next decade, the CBO projects biosimilars will save the U.S. healthcare system $169 billion. That’s not just corporate profit-it’s access. A patient who couldn’t afford Humira at $2,500 a month might now get Hyrimoz for $1,700. That’s the difference between staying on treatment and dropping out.
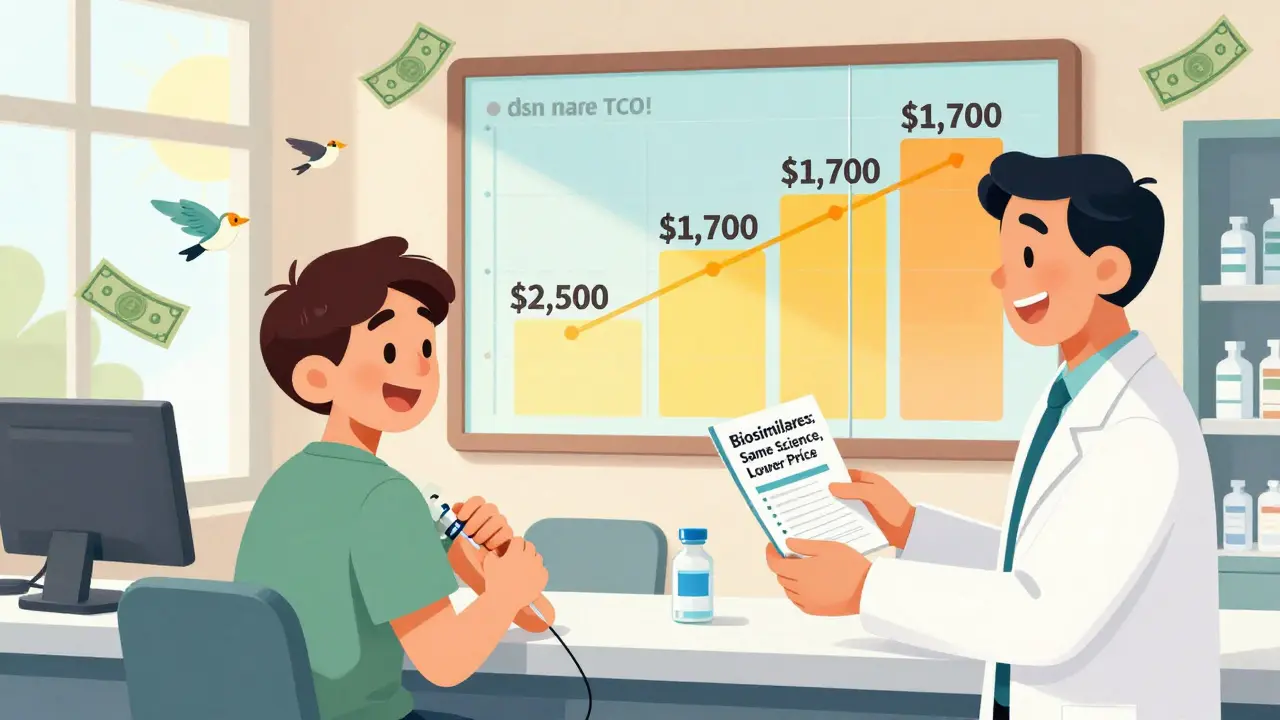
How Are Biosimilars Being Introduced?
Switching isn’t random. Best practices include:
- Provider education-doctors need to understand the approval process
- Patient counseling-explaining that biosimilars aren’t “cheap versions,” but scientifically equivalent
- Electronic health record alerts-so pharmacists know which products are interchangeable
A 2023 study in 15 U.S. health systems showed that with these steps, biosimilar adoption jumped to over 75% within a year. Kaiser Permanente cut patient refusal rates from 22% to just 5% using simple, clear educational materials.
Pharmacists now play a key role. In 48 U.S. states, laws allow pharmacists to substitute interchangeable biosimilars without asking the doctor-similar to how generics work. But only if the product is officially designated as interchangeable by the FDA.
What’s Next for Biosimilars?
The future is getting even better. The FDA is proposing to eliminate the need for clinical trials in cases where analytical and pharmacokinetic data are strong enough. Why? Because over a decade of real-world use has shown no safety or efficacy gaps.
Another trend: switching between biosimilars. A 2023 study found patients who switched from one adalimumab biosimilar to another had the same drug retention rates as those who stayed on one. No drop in effectiveness. No spike in side effects.
And the market is growing fast. The global biosimilar market hit $10.1 billion in 2023 and is projected to reach $38.5 billion by 2030. Major players like Sandoz, Samsung Bioepis, and Amgen are investing heavily. Over 127 biosimilars are in development worldwide.
Bottom Line: Do Biosimilars Work as Well?
Yes. They work as well as the originals. The science is solid. The real-world data is overwhelming. The safety profile matches. The cost savings are undeniable.
That doesn’t mean every patient will respond the same way after a switch. Some might have a flare-up-but that’s true even when staying on the original drug. The difference is, with biosimilars, you get the same chance of control at a fraction of the price.
For patients, the question isn’t whether biosimilars work. It’s whether they can afford to wait for access. For providers, the question isn’t whether they’re effective. It’s whether they’re ready to trust the evidence over the noise.
The data doesn’t lie. Biosimilars aren’t a compromise. They’re a breakthrough.
Are biosimilars the same as generics?
No. Generics are exact chemical copies of small-molecule drugs, like ibuprofen or metformin. Biosimilars are highly similar versions of complex biologic drugs made from living cells. Because biologics are made by living organisms, they can’t be copied exactly-only closely matched. Biosimilars undergo far more rigorous testing than generics to prove they work the same way in the body.
Can I switch from a biologic to a biosimilar safely?
Yes. Multiple studies, including the NOR-SWITCH trial and real-world data from the UK and Canada, show switching from a reference biologic to a biosimilar does not increase the risk of side effects or reduce effectiveness. Most patients report no change in symptoms. Doctors typically monitor patients for 1-3 months after the switch to ensure stability, but this is standard practice regardless of the drug.
Do biosimilars cause more side effects?
No. Studies involving over 500,000 patients show no increase in adverse events with biosimilars compared to the original biologics. Immunogenicity-the risk of the body developing antibodies against the drug-is closely monitored, but real-world data has not shown higher rates of immune reactions with biosimilars. Any flare-ups after switching are more likely due to disease variability than the drug itself.
Why are biosimilars cheaper if they’re just as good?
Because they don’t require the same expensive clinical trials as the original biologic. The reference drug had to go through years of development, massive clinical trials, and marketing to prove safety and efficacy. Biosimilars build on that existing data. They focus on proving similarity, not starting from scratch. This cuts development time and cost, allowing lower prices without sacrificing quality.
Will my insurance cover a biosimilar?
Most do-and many push for it. Insurance companies and Medicare Part B often require patients to try a biosimilar first because of the cost savings. In some cases, they won’t cover the original biologic unless you’ve tried and failed the biosimilar. This is called step therapy, and it’s becoming standard practice.
Can I switch between different biosimilars?
Yes. A 2023 study in Clinical Rheumatology found patients who switched between two different adalimumab biosimilars had the same treatment retention rate as those who stayed on one. There was no drop in effectiveness or increase in side effects. Regulatory agencies now recognize that switching between biosimilars is safe, as long as they’re approved for the same condition.
Are biosimilars approved in the U.S. and Europe?
Yes. The FDA has approved 46 biosimilars as of November 2023, with 37 currently on the market. The European Medicines Agency (EMA) has approved 104 as of December 2023. Both agencies use strict standards to ensure safety and effectiveness. Biosimilars approved in one region are often approved in the other, with similar data requirements.
What if I don’t feel the same after switching?
Talk to your doctor. While most patients report no change, some may experience a flare-up. That doesn’t automatically mean the biosimilar isn’t working-it could be stress, infection, diet, or natural disease fluctuation. Your doctor may recommend staying on the biosimilar for a few more months or switching back. But don’t assume the biosimilar is the cause without evaluation. Studies show that when patients report problems after switching, only a small fraction are actually linked to the drug.




Glenda Marínez Granados
So we’re paying $2500/month for a drug that’s basically a fancy copy… and the pharma companies act like we’re idiots for even considering the cheaper version? 🤡
Meanwhile, my rent went up 40% and my insulin still costs more than my phone bill. Biosimilars aren’t ‘compromises’-they’re the only thing keeping me alive. Thanks, capitalism.
MARILYN ONEILL
OMG I can’t believe people are actually okay with this. It’s not the same! You can’t just swap out a life-changing drug like it’s a different brand of toilet paper. What if it doesn’t work? What if I get worse? This is MY BODY we’re talking about!!
Steve Hesketh
Bro, I’ve been on a biosimilar for 3 years now after switching from Humira. No flare-ups. No weird side effects. Just the same relief, at half the cost.
My mom in Nigeria couldn’t even get the original-she’d have to sell her land. Biosimilars aren’t just smart science-they’re justice. People like us? We’re not lucky. We’re surviving because someone finally said, ‘Enough.’ Keep fighting for access. You’re not alone.
Kevin Narvaes
so like… the whole system is rigged right? like we’re supposed to trust these drugs because the FDA says so… but who even is the FDA? some corporate puppet? i mean… i’ve seen the ads for the originals… they’re like, ‘trust us, we’re scientists’… but then they charge 10k a month??
it’s not about science… it’s about control. we’re all just lab rats with insurance cards.
Dee Monroe
It’s funny how we treat medicine like it’s some sacred ritual, you know? Like if it’s not the exact same bottle with the same logo, it’s not ‘real’ healing. But the body doesn’t care about branding. It cares about molecules, pathways, receptor binding. If the structure, the half-life, the cytokine suppression-all of it-is statistically identical, then the difference is psychological, not pharmacological.
And honestly? The fear of switching isn’t about the drug. It’s about losing control. We’ve been conditioned to believe that expensive = better, that brand = safety, that paying more means you’re doing something right. But medicine isn’t luxury fashion. It’s biology. And biology doesn’t care about your brand loyalty.
That’s why the real revolution isn’t in the lab-it’s in the mind. We have to stop worshipping the original and start trusting the evidence. The data doesn’t lie. The people who’ve switched? They’re not guinea pigs. They’re pioneers. And if we keep letting fear dictate treatment, we’re not just wasting money-we’re wasting lives.
Alex Carletti Gouvea
Why are we letting foreign companies make our life-saving drugs? America should be producing these. This is national security. Biosimilars are just a Trojan horse for outsourcing. We need American-made biologics. Period.
Philip Williams
Thank you for this comprehensive breakdown. The data is unequivocal: biosimilars are clinically equivalent. What’s more impressive is the scale of real-world validation-over half a million patients across multiple continents, with no safety signal. The regulatory rigor applied to biosimilars is actually more intensive than for many generics. This isn’t a cost-cutting shortcut-it’s a triumph of evidence-based medicine.
What’s concerning is the persistence of misinformation among clinicians. Education must be prioritized. Pharmacists, too, need clear guidelines for substitution. We’re not just saving money-we’re expanding access to care for millions who otherwise go untreated. This is public health at its best.
Jerry Rodrigues
Switched to Hyrimoz last year. No difference. Still alive. Still working. Still not broke.
Done.
lokesh prasanth
data says yes but what about the 3% who get worse? they dont count? the system ignores them. biosimilars are a numbers game. people are just stats.