Drug Interaction Checker for Acid Reducers
Check Medication Interactions
Enter your medications below to see if acid-reducing drugs might affect their absorption.
Results
When you take a proton pump inhibitor like omeprazole or a histamine blocker like famotidine for heartburn, you’re not just changing your stomach’s acidity-you might be quietly sabotaging the effectiveness of other medications you’re taking. This isn’t theoretical. It’s happening right now to people who don’t even know it’s possible. In fact, acid-reducing medications interfere with the absorption of at least 15 commonly prescribed drugs, sometimes dropping their effectiveness by more than 90%.
How Acid-Reducing Medications Work
Proton pump inhibitors (PPIs) and H2-receptor antagonists (H2RAs) do the same basic job: they lower stomach acid. PPIs like omeprazole, esomeprazole, and lansoprazole shut down the acid-producing pumps in stomach cells. H2RAs like ranitidine and famotidine block histamine signals that tell those pumps to turn on. Both raise gastric pH from its normal range of 1-3.5 to around 4-6. That’s a big shift. It’s like turning a chemical reactor from a strong acid bath into a mild vinegar solution.
For people with ulcers or GERD, this helps. But for drugs that rely on stomach acid to dissolve properly, it’s a disaster. Most people assume that if a pill gets swallowed, it’ll be absorbed just fine. That’s not true. The way a drug dissolves in your gut determines whether your body can use it at all.
Why pH Changes Break Drug Absorption
Many drugs are weak bases-meaning they need an acidic environment to dissolve. Think of them like salt: they only dissolve well in water that’s acidic enough. When your stomach pH rises, these drugs stay solid instead of dissolving. They pass through your system unchanged. This isn’t a minor issue. About 70% of oral medications are weak bases, according to drug databases analyzed in 2024. That includes HIV meds, cancer drugs, antifungals, and even some blood pressure pills.
The science behind this is the Henderson-Hasselbalch equation. It sounds complicated, but it’s really just about ionization. A drug with a pKa above 7 (like atazanavir) stays neutral in acid, which lets it cross cell membranes. But in a less acidic stomach, it becomes charged and can’t dissolve. No dissolution = no absorption. And if the drug doesn’t get absorbed, it doesn’t work.
Drugs Most Affected by Acid Reducers
Some drugs are hit harder than others. Here are the big ones:
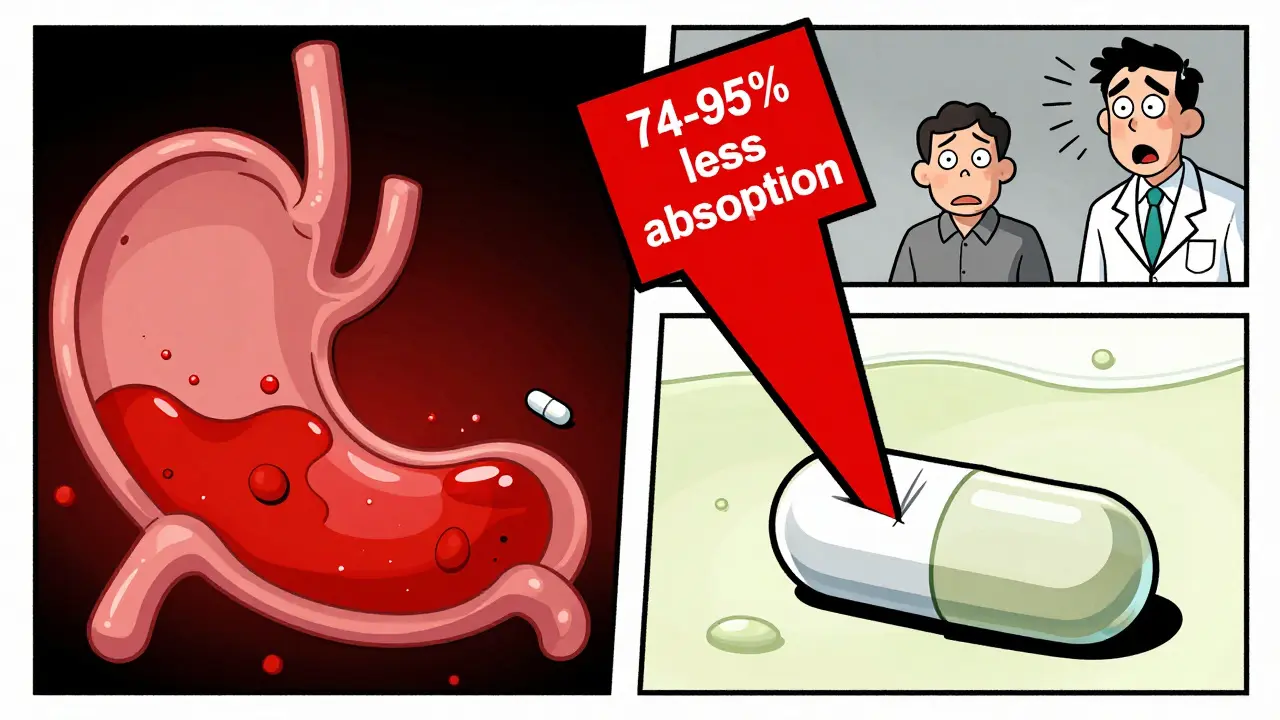
- Atazanavir (HIV treatment): AUC drops by 74-95% when taken with a PPI. That’s not just reduced effectiveness-it’s treatment failure. Viral loads can spike from undetectable to over 10,000 copies/mL.
- Dasatinib (leukemia drug): Absorption drops by 60%. Patients can relapse because the drug never reaches therapeutic levels.
- Ketoconazole (antifungal): 75% less absorption. It becomes useless against fungal infections.
- Nilotinib (another leukemia drug): Absorption drops sharply with PPIs, requiring dose adjustments or complete avoidance.
- Myfortic (mycophenolate mofetil): Used after organ transplants. Reduced absorption increases rejection risk.
Even common drugs like iron supplements and certain thyroid meds (levothyroxine) can be affected. The FDA lists 12 high-risk drugs that now require specific warnings on their labels because of these interactions.
PPIs vs. H2RAs: Not All Acid Reducers Are Equal
Not every acid-reducing drug is the same. PPIs are the bigger problem. They suppress acid for 14-18 hours a day. H2RAs last only 8-12 hours. That means PPIs create a longer window for drugs to fail.
A 2024 study in JAMA Network Open found PPIs reduce absorption of pH-dependent drugs by 40-80%. H2RAs? Only 20-40%. That’s a huge difference. If you’re on a high-risk drug like atazanavir, even an H2RA might be too risky. But if you’re on something less sensitive, switching from a PPI to an H2RA might be a compromise.
Also, formulation matters. Immediate-release pills dissolve fast and are more vulnerable. Extended-release versions may be less affected because they release slowly over time, bypassing the worst of the pH shift.
What About Acidic Drugs?
Not all drugs are weak bases. Some are weak acids-like aspirin or ibuprofen. These actually dissolve better in higher pH environments. But here’s the catch: most of these drugs are absorbed in the small intestine, not the stomach. So even if they dissolve better in a less acidic stomach, it doesn’t mean more gets absorbed. In fact, for most acidic drugs, the change is minimal-usually less than 25% increase in absorption. That’s rarely clinically meaningful.
There’s one exception: dasiglucagon, used for severe low blood sugar. It shows a slight increase in absorption with ARAs, but not enough to require dose changes. So while acidic drugs aren’t safe from interactions, they’re not the main concern.
Real-World Consequences
This isn’t just a lab phenomenon. It’s happening in living rooms, hospitals, and pharmacies.
A 2023 study of over 12,500 patients found that those taking dasatinib with a PPI had 37% more treatment failures than those who didn’t. In Reddit threads, patients describe viral rebound after starting omeprazole for heartburn. One wrote: “My viral load went from undetectable to 12,000 copies/mL after starting Prilosec.”
Another user on Drugs.com said: “My doctor didn’t tell me Nexium would interfere with my blood pressure meds. My readings were 20 points higher until we figured it out.”
The FDA’s adverse event database recorded over 1,200 reports of therapeutic failure linked to acid-reducing meds between 2020 and 2023. The top three culprits? Atazanavir, dasatinib, and ketoconazole.
How to Fix It
You can’t just stop taking your heartburn meds if you need them. But you can manage the risk.
- Stagger doses. Take the affected drug at least 2 hours before the acid reducer. For weak bases, this helps-though it only cuts the interaction by 30-40%, not enough to fully fix it.
- Use antacids instead. Tums or Maalox work fast and don’t last long. Take them 2-4 hours apart from your other meds. But they’re not good for daily use.
- Switch to an H2RA. If you’re on a PPI and need to keep acid reduction, ask your doctor about switching to famotidine. It’s less disruptive.
- Ask about alternatives. Is there a different HIV drug that doesn’t interact? A different cancer med? Sometimes there is.
- Check your pharmacist. A 2023 study showed pharmacist-led reviews cut inappropriate ARA co-prescribing by 62%. Pharmacists see your full list. They’re trained to catch these.
Some hospitals now use electronic alerts in their systems. If you’re on atazanavir and your doctor tries to prescribe omeprazole, Epic or Cerner will pop up a warning. But not all systems are set up that way. Don’t rely on tech alone.
Who’s at Risk?
Anyone taking chronic acid-reducing meds is at risk-especially if they’re also on:
- HIV antivirals
- Cancer drugs (especially tyrosine kinase inhibitors)
- Antifungals
- Immunosuppressants
- Thyroid meds
- Iron or calcium supplements
And here’s the scary part: about 15% of adults in developed countries take these drugs long-term. The American College of Gastroenterology says 30-50% of those prescriptions are unnecessary. People take them for occasional heartburn, or because their doctor didn’t reassess them in years. That’s a massive population at risk for hidden drug failures.
What’s Changing?
The FDA released new guidance in 2023 requiring drug makers to test new medications for pH-dependent interactions. They now mandate testing across pH levels from 1.0 to 7.5. If a drug dissolves poorly above pH 5 and has a narrow therapeutic index, it must carry a warning.
Pharma companies are responding. About 37% of new drugs in development now use special coatings or delivery systems to avoid pH dependence. Some are even being designed to work without needing stomach acid at all.
AI tools are also emerging. Google Health’s prototype predicts drug interactions with 89% accuracy by modeling pH, solubility, and patient physiology. That’s not in clinics yet-but it’s coming.
Meanwhile, deprescribing initiatives are gaining ground. The American Gastroenterological Association expects a 25% drop in inappropriate PPI use by 2027. That could prevent 5,000-7,000 cases of therapeutic failure every year.
What You Should Do
If you’re taking an acid-reducing medication and another drug that’s critical to your health:
- Ask your doctor: “Could this be affecting how my other meds work?”
- Ask your pharmacist to run a full interaction check on all your pills.
- Don’t assume “it’s just heartburn” means it’s harmless.
- Don’t stop your acid reducer without talking to your provider-but do question whether you still need it.
Many people take PPIs for years without ever being re-evaluated. But if you’re on a cancer drug, HIV treatment, or transplant medication, this interaction could be life-threatening. And it’s completely avoidable-if you know to look for it.
Can acid-reducing medications make my other drugs completely useless?
Yes, in some cases. For example, combining the HIV drug atazanavir with a proton pump inhibitor can reduce its absorption by up to 95%. That means the drug doesn’t reach the level needed to suppress the virus, leading to treatment failure and possible drug resistance. Similar effects occur with cancer drugs like dasatinib and antifungals like ketoconazole. These aren’t minor reductions-they’re clinically significant failures.
Are all acid-reducing drugs equally risky?
No. Proton pump inhibitors (PPIs) like omeprazole and esomeprazole suppress acid for 14-18 hours a day, making them far more likely to interfere with drug absorption. H2-receptor antagonists like famotidine or ranitidine only work for 8-12 hours and cause less pH elevation. While H2RAs are still risky with certain drugs, they’re often a safer choice if you need ongoing acid reduction. Antacids like Tums have minimal lasting effect and can be used with more flexibility if timed correctly.
How do I know if my medication is affected?
Look for drugs that are weak bases with low solubility at higher pH levels. Common examples include atazanavir, dasatinib, ketoconazole, mycophenolate, and levothyroxine. Check the drug’s prescribing information for warnings about “gastric pH” or “acid-reducing agents.” If you’re unsure, ask your pharmacist to run a full drug interaction check. Many pharmacies now offer this service for free.
Can I just take my other meds at a different time of day?
Timing helps, but it doesn’t fix everything. Taking your weak base drug 2 hours before your PPI can reduce the interaction by about 30-40%. That’s better than nothing, but it won’t restore full absorption. For drugs like atazanavir, even staggered dosing isn’t enough-you need to avoid PPIs entirely. For less critical drugs, timing might be a practical compromise. Always check with your doctor or pharmacist before changing your schedule.
Should I stop taking my acid reducer if I’m on other meds?
Don’t stop abruptly. If you’re taking an acid reducer for a real medical reason-like a bleeding ulcer or severe GERD-you need to keep it. But ask your doctor if you still need it at all. Studies show that 30-50% of people on long-term PPIs don’t have a valid indication. If your heartburn is mild or occasional, you might be able to stop or switch to an H2RA or antacid. Never make this decision alone-work with your provider to weigh the risks and benefits.
If you’re managing multiple medications, especially for chronic conditions, don’t assume your pills work in isolation. The chemistry in your stomach matters. A simple heartburn med could be quietly undermining your cancer treatment, HIV control, or transplant success. The fix isn’t always complicated-but you have to ask the right questions.



sagar sanadi
lol so now even my tums is a CIA tool? next they'll say aspirin is a mind control chip. 🤡
kumar kc
People take PPIs like candy. No wonder we're drowning in drug failures.
clifford hoang
This is all part of the Pharma Deep State. They want you dependent on meds so they can sell you more. PPIs? They're just the gateway drug to lifelong corporate slavery. 💉📉 #WakeUp
Jacob Cathro
so like... if i take my cancer meds at 6am and my prilosec at 8pm... is that cool? or am i just gonna die quietly? 😭
Paul Barnes
The pharmacokinetic implications of altered gastric pH on weakly basic pharmaceuticals are well-documented. This is not speculative. It's biochemistry.
pragya mishra
Wait, so you're telling me my uncle's heartburn pill could be killing his chemo? Why didn't his doctor tell him? I'm calling his doctor right now.
Manoj Kumar Billigunta
I've been taking famotidine for years and my dad's on HIV meds. I always told him to take his pills 2 hours before the heartburn stuff. Glad I didn't need a study to know that. Stay smart, folks.
Andy Thompson
America's biggest problem? People taking drugs for heartburn because they eat too much McDonald's. Fix your diet, not your stomach acid. 🇺🇸🍔 #StopThePPIs
thomas wall
One cannot help but observe with profound dismay the alarming normalization of pharmacological ignorance among the general populace. The erosion of medical literacy is not merely regrettable-it is catastrophic.
Arlene Mathison
This is why you need to be your own advocate! Don't wait for your doctor to catch it-ask your pharmacist. You got this! 💪❤️
Emily Leigh
ok but like... if i take a PPI and then my blood pressure med... does that mean my house is gonna fall down? or is that just a metaphor? 😅😅😅
Carolyn Rose Meszaros
I had no idea this was even a thing... my grandma's on 7 meds and takes Tums daily. I'm sending her this right now. 🙏❤️
Greg Robertson
Thanks for sharing this. I'm gonna ask my pharmacist to check my med list next time I refill. Better safe than sorry, right?
Crystal August
This is why I don't trust doctors. They're all in bed with Big Pharma. If they really cared, they'd tell you to eat kale and stop being lazy.
Nadia Watson
While the scientific literature robustly supports the clinical significance of pH-dependent drug interactions, it is imperative to acknowledge the systemic barriers to patient education. In underserved communities, access to pharmacy consultations remains uneven, and this disparity exacerbates preventable therapeutic failures. The solution lies not only in clinical awareness but in equitable healthcare infrastructure.