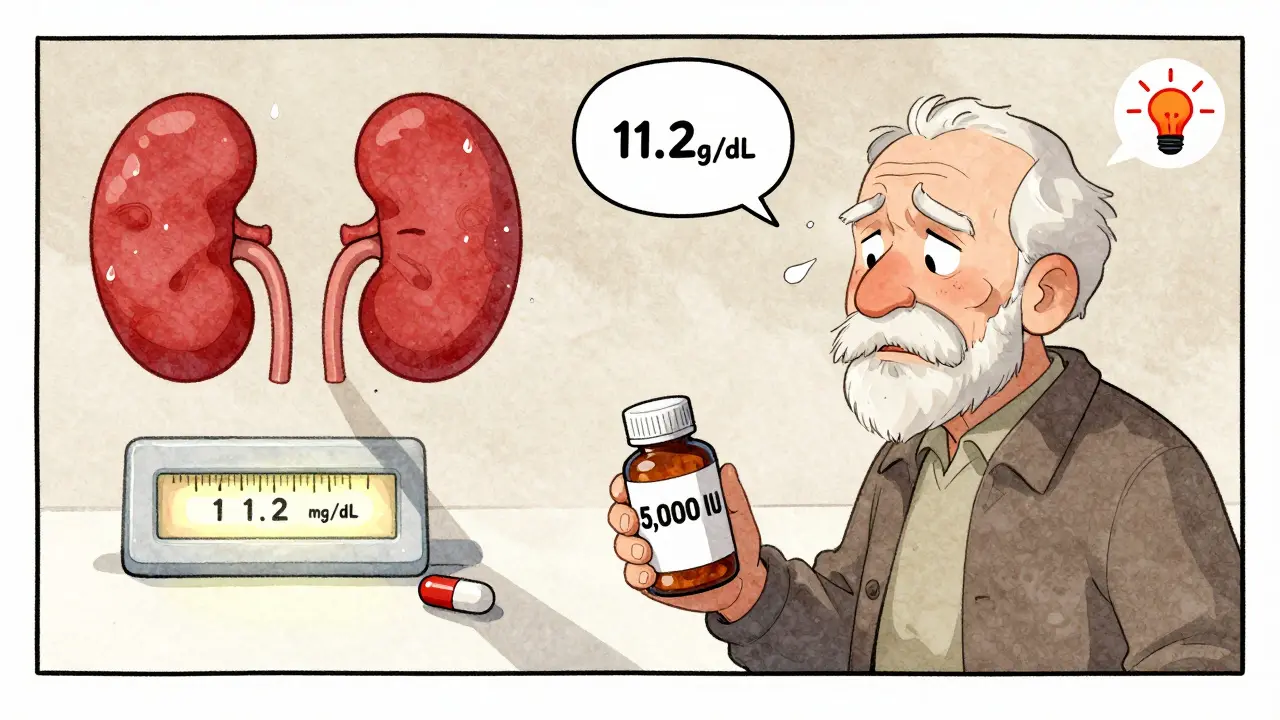
Vitamin D and Thiazide Diuretics: What You Need to Know About Hypercalcemia Risk
Taking vitamin D with thiazide diuretics can raise blood calcium to dangerous levels. Learn how this interaction works, who’s at risk, and what steps to take to stay safe without stopping either medication.
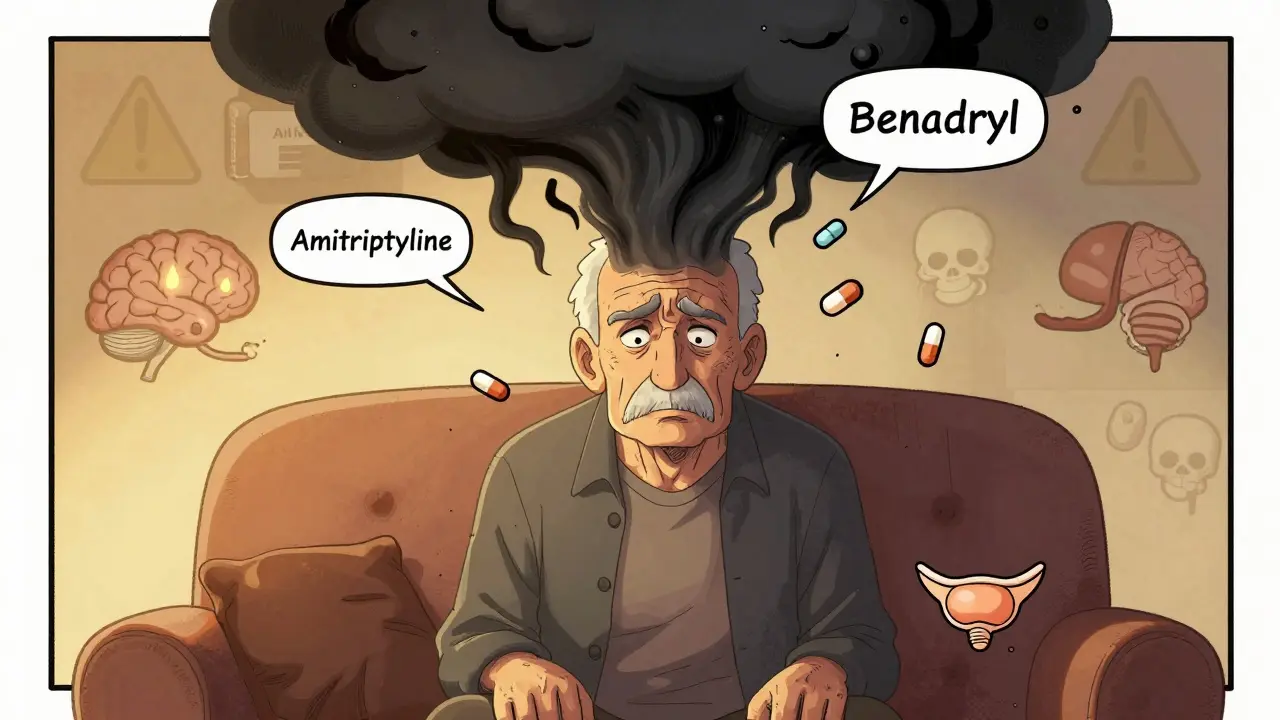
Read MoreTricyclic Antidepressants and Antihistamines: The Hidden Danger of Anticholinergic Overload
Combining tricyclic antidepressants like amitriptyline with first-gen antihistamines like Benadryl can cause dangerous anticholinergic overload-leading to confusion, memory loss, and increased dementia risk. Learn how to spot it and switch to safer options.
Read MoreHow to Prevent Early Refills and Duplicate Therapy Mistakes in Pharmacy Practice
Learn how pharmacists can prevent dangerous early refills and duplicate therapy mistakes using proven protocols, technology, and patient-centered communication to improve medication safety and reduce harm.
Read MoreAlternative Cholesterol Medications: Ezetimibe and Bempedoic Acid for Statin-Intolerant Patients
Ezetimibe and bempedoic acid offer effective, oral alternatives for people who can't tolerate statins. Both lower LDL cholesterol with fewer muscle side effects, and bempedoic acid has proven heart benefits in large trials.
Read MoreTherapeutic Equivalence Codes (TE Codes) Explained: How Generic Drugs Are Approved and Substituted
Therapeutic Equivalence Codes (TE codes) are the FDA's system for determining which generic drugs can safely replace brand-name medications. Learn how they work, why they matter for your prescriptions, and when substitutions might not be ideal.
Read MoreCommon Pharmacy Dispensing Errors and How to Prevent Them
Discover the most common pharmacy dispensing errors, why they happen, and proven ways to prevent them. Learn how barcode scanning, double-checks, and smart systems reduce medication mistakes and protect patients.
Read MoreEuglycemic DKA on SGLT2 Inhibitors: How to Recognize and Treat This Hidden Emergency
Euglycemic DKA on SGLT2 inhibitors is a hidden emergency that strikes with normal blood sugar. Learn the symptoms, why it happens, and how to treat it before it’s too late.
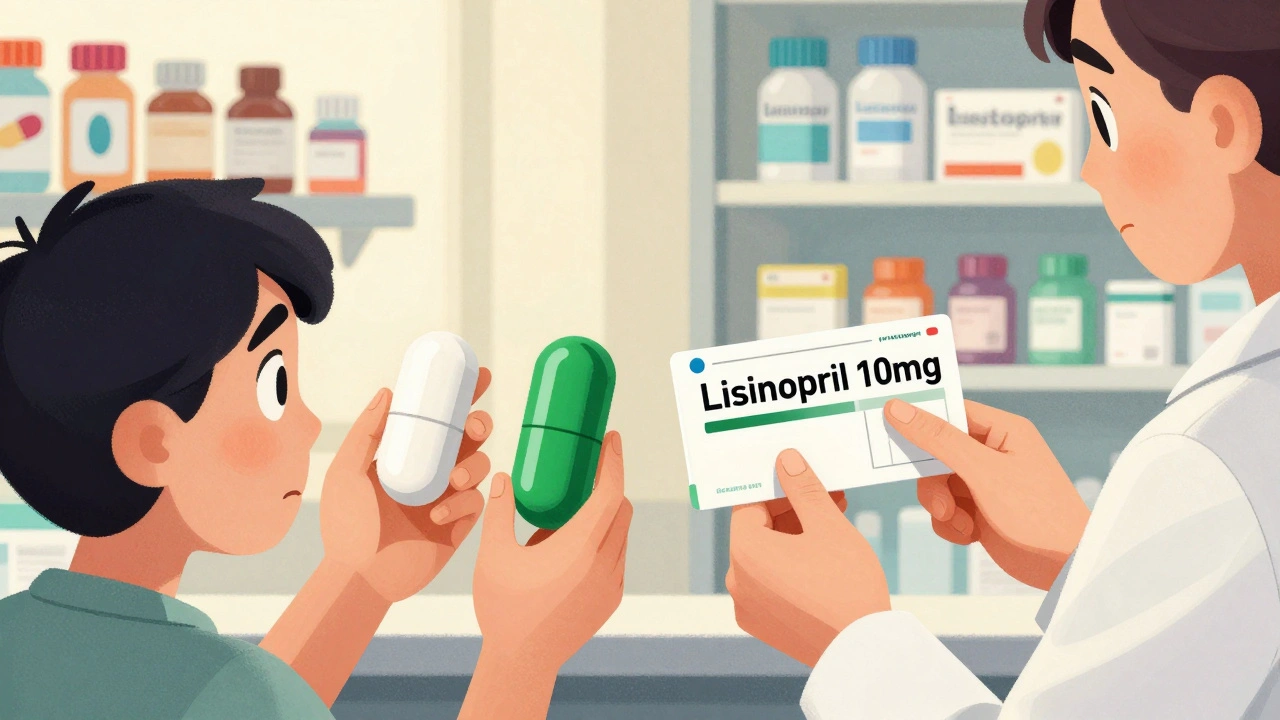
Read MoreWhy Generic Drugs Look Different: The Role of Trademark Laws
Generic drugs look different from brand-name versions because U.S. trademark laws require visual distinction to prevent consumer confusion - not because they're less effective. Learn why color, shape, and size change, and how to stay safe when your prescription looks unfamiliar.
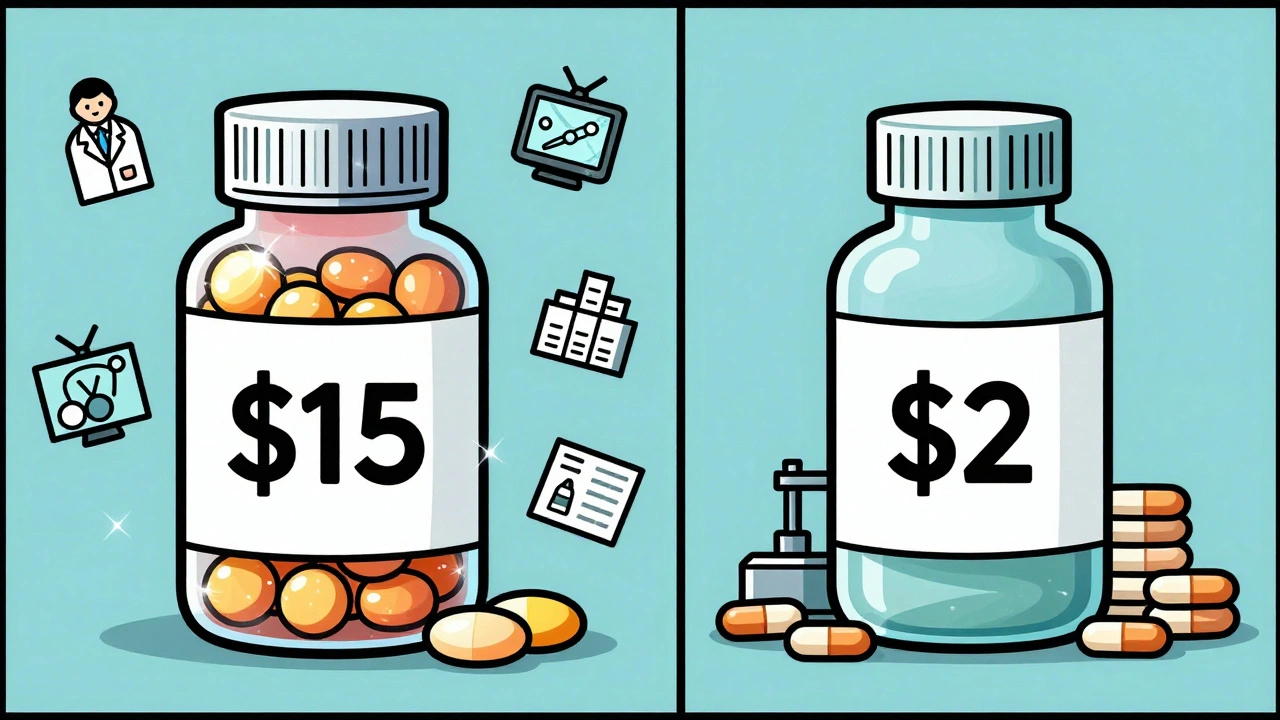
Read MoreManufacturing Cost Analysis: Why Generic Drugs Are So Much Cheaper
Generic drugs cost far less than brand-name versions because they skip expensive R&D and marketing. Manufacturing at scale, using proven formulas, and avoiding patent costs makes generics up to 95% cheaper - saving billions in healthcare spending.
Read MoreComplex Generic Drugs: Why Some Products Are Harder to Get FDA Approval
Complex generic drugs like liposomal injections and inhalers face major FDA approval hurdles due to scientific, technical, and regulatory challenges - making them far harder to bring to market than simple generics.
Read More